Research Article - (2021) Volume 9, Issue 3
Crosslinking of chitosan with eri silk using maleic acid
Ashis Narayan Amita Banerjee1, Dibyendu Bikash Datta2*, Rajiv Munshi3 and Debasish Das12National Institute of Fashion Technology, Ministry of Textiles, Kolkata, India
3Central Silk Board, Ministry of Textiles, India
Abstract
Polycarboxylic acid compounds such as butane tetra carboxylic acid (BTCA), cyclopentane tetracarboxylic acid, (CPTA), and citric acid offer an environmentally friendly, non-toxic, and safe alternative to the use of toxic formaldehyde condensate resin as a crosslinking agent for silk. However, the sodium salts of phosphoruscontaining mineral acids used as esterification catalysts with such polycarboxylic acids are not environmentally friendly in view of their reported adverse effects on the aquatic environment and soil. Also, finishes based on such non-polymeric polycarboxylic acids cannot retain or improve the strength and moisture-regain characteristics of silk. Moreover, most polycarboxylic acids are too expensive for practical exploitation. In view of the above, the present work was aimed at establishing the optimum condition for the application of maleic acid on silk fabric in the presence of ammonium persulphate as the free radical polymerization catalyst and trisodium citrate as the esterification catalyst. Water-soluble chitosan with varied concentrations of 0.5-2 g/L was also incorporated in the finishing bath to impart antibacterial activity to the fabric along with the easy-care characteristics. Evaluation of attainable changes or improvements in the silk fabric properties in respect of tensile strength, wrinkle recovery, flexibility, antimicrobial and moisture regain on such treatments have been done. Also, changes in the chemical nature of silk fabric on such modifications have been studied by infrared spectroscopy and reported in the article. The study recommends the thermal curing system is conducive to the production of chitosan and maleic acid-treated eri silk fabrics with antibacterial and easy-care properties, without a significant loss in strength properties.
Keywords
Maleic acid, Curing, Ammonium persulphate, Trisodium citrate, Esterification, Free-radical polymerization
Introduction
Silk is an important environment-friendly biodegradable protein fiber considered and identified to be the textile fiber of the future that supports most among the natural fibers the growing concept of sustainably in respect of its production from the silkworm. Silk fiber also has some other advantages viz. Its high strength, appreciable moisture regain, low specific gravity, appreciable elastic recovery, and good thermal stability. Silk is primarily appreciated for its luster, elegant appearances, and soft feel particularly when the soluble globular protein or gum as it is called is removed from linear protein macromolecules of silk fiber. The draw black of silk fiber lies in its poor crease resistance and also susceptibility to attack by microbial organisms. Out of the four types of silk, eri silk, also known as the silk of the poor is majorly found in the northeast of India. The woolly white silk is often termed as errandi or endi silk in India. The name "eri" is derived from the Assamese word ‘era’, which means ‘castor’, as the silkworm feeds on castor plants.
Eri silk is one of the purest forms of silk that is a true and genuine product of the samia cynthia ricini worm. Eri silk is called the father of all forms of cultured and textured silks. It is the only domesticated silk produced in India, as the process does not involve any killing of the silkworm. The process results in a silk called ‘ahimsa’ silk or fabric of peace (Deshmukh, 2009). Nevertheless, this eri silk has excellent qualities. It is very strong, combining the elegance of silk with the comfort of cotton and warmth of wool (Das et al., 2017). Eri silk is the most textured silk that needs a huge amount of preservation and care strategy. It has shorter fibers than the usual cultured silks. The shorter fibers of eri silk make it less durable. It is indeed one of the softest and purest forms of silk which is fancied by almost all the silk lovers' wardrobe. The silkworms give the eri silk a dull yellow, gold like sheen. However, apart from the apparel usage eri silk has also usage for functional textiles.
Crease resistance finishing of cotton and silk textile using resins from amine formaldehyde condensates such as dimethylol dihydroxy ethylene urea (DMDHEU), dimethylol propylene urea (DMPU), resins result in some odd disadvantages in respect of relatively poor tensile strength retention despite significant improvement in wrinkle recovery (Lee and Sin, 1991; Yang and Li 1992). Such amine formaldehyde condensate resin finishes are also associated with the disadvantage of formaldehyde splitting during processing and use, endangering the health of processors and species and also as a probable carcinogen. Finishing of cotton and silk with polycarboxylic acids as formaldehyde-free finishing agents such as, butane tetra carboxylic acid (BTCA), cyclopentane tetracarboxylic acid (CPTA) appear to be much more prospective in this respect. Such compounds have evoked immense interest in the recent past in view of their environment-friendly and nontoxic characters. However, such compounds are too expensive to be practically exploited and not widely available. Silk fabrics are often subjected to chemical finishing using crosslinking agents to convey easy-care properties. Amine formaldehyde condensate resin finishes are also associated with the disadvantages of formaldehyde splitting during processing and use, endangering the health of processors and users (Munshi et al., 2014). Though the crosslinks contributed towards fabric wrinkle resistance, also resulted in discoloration and impairment of fabric strength and other mechanical properties crosslinking between phenolic (-OH) and primary hydroxyl groups, respectively of tyrosine and serine amino acids of silk macromolecular network causes fiber embrittlement that reduces the treated fabric's mechanical strength(Kang et al., 1998; Das and Munshi 2006).
Also, sodium salts of phosphorous-containing mineral acid used as an esterification catalyst with such polycarboxylic acid compounds are not environment friendly (Brodmann, 1990; Cao, 2000). Such catalysts contain phosphorous influence reproduction of fish and favors growth of a kind of seaweed which consumes a large amount of oxygen from water giving rise to what is called eutrophication. Also, finishes based on such nonpolymeric polycarboxylic acid cannot retain and/or improve strength and moisture regain characters of cotton and silk (Das et al., 2011; Das et al., 2015). However, the report of the effect of the polymerizable vinyl monomer for improvement in wrinkle recovery of cotton and silk fabrics are scanty. Carboxyl containing vinyl monomer like maleic acid under the influence of an appropriate catalytic system shall produce anti-crease finishing on silk substrate (Shirvan et al., 2019).
From beginning to end; being at the forefront of both technical and artistic progress, textiles have always played a central role in the evolution of human culture. Textile safety features have paved the foundation for ground breaking innovations. Textiles have such a significant impact on our everyday lives that everyone wants to know about them. People have used textiles of various types from the earliest times for protection, comfort, personal ornamentation, and even for displaying personal property. Of these reasons, textiles are still being used today and everybody is the main buyer. Consumers are now increasingly aware of the hygienic way of life, and a wide range of textile products with antimicrobial finishes are required and anticipated.
People have been more concerned with their fitness, grooming, lifestyle, fashion, convenience, luxury and wellness over the last few decades. Many belonging to economically well-off sections more frequently prefer to buy luxury textiles, not only to show their high social and well-off strata, but also from the compulsion of the present day in terms of health and hygienic need. To establish aristocratic, social, religious, business, and ritual party outfit, the requirement of various qualities for professional clothing such as comfort, hygiene, and fabric surface self-cleaning was integrated in textiles. High-value cosmeto-textile and ayurvastra have also been built for various diseases to slim, moisturize, perfume, look good, new, and cure. Considering all these high-value textiles are made primarily from natural fibers and are functionalized using natural products/biomaterials to be used effectively to manufacture sustainable luxury textiles with anti-bacterial properties. The natural fibers silk, silk, and wool with good antibacterial properties are having usage in medical textiles, baby dress materials, health workers' uniforms, hospital linens, sanitary items, N95 masks for giving protection against dust particles and airborne germs and many more. The N95 masks, wipes, and towels with anti-microbial properties became more relevant in view of the present perspective when the entire world is combating Corona (COVID-19) virus.
The concept of ‘antibacterial finishing for textiles’ has made significant advances in developing antibacterial fibers and agents in the textile industry. An ideal silk textile antibacterial finishing should not only kill undesirable microorganisms and stop the spread of diseases, but also fulfill three other basic requirements (Arai et al., 2001). First, safety: the product should not be excessively toxic to humans and the environment and should not cause skin allergy and irritation. Second, compatibility: the product must not present negative influences to textile properties or appearances and must be compatible with common textile processing. Third, durability: the product should be able to endure laundering, drying, and leaching. Researchers are now focusing on safe, durable, and environment-friendly natural substitutes as finishing agents.
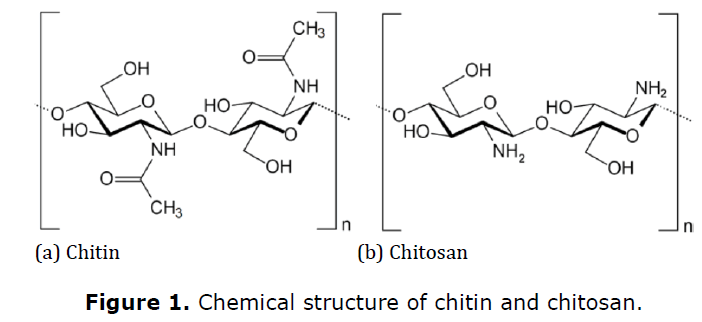
Chitin that is more than 55% deacetylated and called as chitosan, is a biopolymer with unique properties such as biodegradability, nontoxicity, and antimicrobial activity (Figure 1a and 1b). Chitosan is already used in textile field for anionic dyeing and finishing, but the major problems of chitosan as an antimicrobial agent are the activity loss under alkaline conditions due to modification of the cationic nature and the poor durability on textiles due to lack of strong bonding with fabrics (Lim and Hudson 2004; Sudarshan et al., 1992; Tsai and Su 1999; Shin and Min 1996). Hence, it is applied by wet thermal curing involving relatively high temperatures with energy consumption, higher costs, and possible fabric degradation. Moreover, the addition of crosslinking agents is required (El-Tahlawy et al., 2005; Alonso et al., 2009).
Chitosan has a high molecular weight and inter and intra molecular hydrogen bonding which makes it chemically stable and of low water solubility (El-Shafei et al., 2008). Chitosan is soluble only in certain dilute acid solutions which limits its wider use. Chitosan can be hydrolyzed into low molecular weight oligosaccharides (Munshi and Majumdar 2018). The molecular structure of chitosan is similar to that of cellulose except for the hydroxyl groups on the carbon atom number two which have been replaced by amino groups. Chitosan can be applied as a silk finish to produce fabrics with properties similar to cellulose fabrics (Figure 1a) (Bang et al., 2007). This has become an area of increased research interest.
Chitin and its deacetylated derivative chitosan are natural polymers composed of randomly distributed β-(1-4)-linked d-glucosamine (deacetylated unit) and n-acetyl-d-glucosamine (acetylated unit). Biopolymers like chitin and chitosan exhibit diverse properties that open up a wide-ranging of applications in various sectors especially in biomedical science. The latest advances in biomedical research are important emerging trends that hold a great promise in wound-healing management products. Chitin and chitosan are considered useful biocompatible materials to be used in a medical device to treat, augment, or replace any tissue, organ, or function of the body.
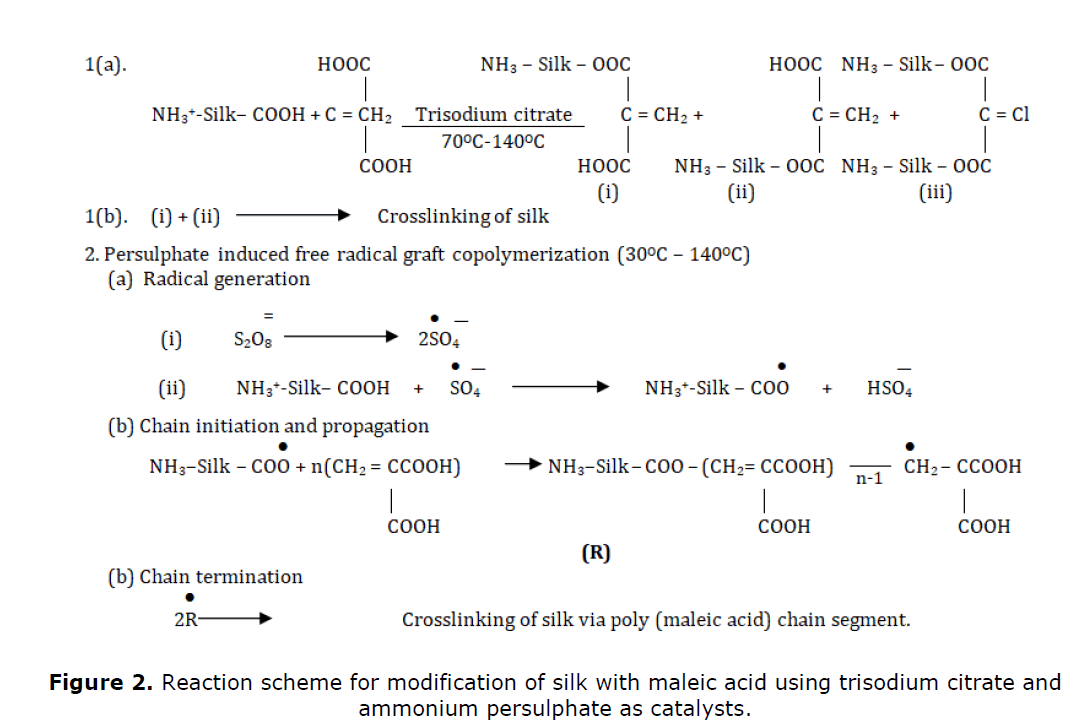
In this context, it would be useful if we consider the mechanism of the intended modification of eri silk with maleic acid following the pad-dry-cure technique under the influence of ammonium persulphate used as the free radical polymerization catalyst and trisodium citrate used as esterification catalyst (Das et al., 2014).
Hydroxyl groups of amino acid of silk are expected to bring about intended modifications under the sequence of reactions shown in Figure 2. Such intended modification of silk fiber ultimately would lead to a notable gain in weight and changes in the chemical nature and physical properties of silk during the overall process. Reaction 1 producing maleic acid esters of silk would be the direct consequence of the action of trisodium citrate used as an esterification catalyst. The said esterification reaction would also expectedly lead to crosslinking of silk as mentioned in the reaction scheme 1. However maleic esters of silk as shown by the structures (i) and (ii) may then react further with hydroxyl groups of silk respectively leading subsequently to the linking of silk via an ester bridge formed by the maleic acid moiety as shown by reaction 1 during the drying and curing step.
Influence of ammonium persulphate as free radical catalyst on the other hand in pad-dry-cure technique would cause graft copolymerization of maleic acid under the treatment condition ultimately leading to the grafting of poly (maleic acid) chain on the chain molecule of silk under the treatment condition with ultimate crosslinking of silk in addition to peroxodisulphate induced free radical homopolymerization of maleic acid; not shown in the scheme) is another distinct possibility. Such peroxodisulphate induced graft copolymerization and crosslinking would cause enhancement of carboxylic group content of the maleic acid-modified silk via maleic acid and improvement in wrinkle recovery of silk inconsequent to expected crosslinking of polymeric chains of silk. Under the influence of two catalysts taken together for the maleic acid curing of silk, all the reactions shown and discussed above are likely to take place simultaneously or successively Moreover, additional reactions leading to further graft copolymerization and esterification with consequent eventual complex network formation involving further unreacted hydroxyl groups of silk and also carboxyl groups and unsaturation of poly (maleic acid) moieties duly grafted to silk may also take place under the treatment condition leading to improved wrinkle recovery of maleic acid finished silk.
The microorganism which is unicellular in leading to weight gain for the fabric system.
structure used to grow at a rapid pace under warmth and moisture. It grows rapidly in the presence of humidity, heat, and food sources whether it is Gram-positive (Staphylococcus aureus) and Gram-negative (Klebsiella pneumoniae). The human skin is very conducive for bacterial growth due to the acidic or basic nature of perspiration. After antimicrobial finishing, silk fibers inhibit a supportive environment for microbial growth (Munshi and Majumdar 2018). The present work was undertaken to thermal curing of eri silk fabrics treated with maleic acid as formaldehyde-free finishing agent along with water-soluble chitosan to impart easy care and antimicrobial properties. Finishing agents comprised of trisodium citrate and ammonium persulphate as catalyst. This research article is mainly focused on the contemporary research on the usage of chitosan on silk towards their applications in numerous biomedical fields’ namely surgical gowns, drapes, wound-healing, burn treatment, and some other useful purposes in the present scenario of COVID-19 pandemic outbreak worldwide.
Experimental
Materials
Eri silk fabric with warp count 32 Ne (18 Tex) and weft count 30 Ne (20 Tex) having an average area density of 88 gm2 was used for the present study. In this experiment generally we use silk, degummed eri silk fabric. Commercial grade maleic acid obtained from M/s Macromols India Ltd and commercial grade water-soluble chitosan obtained from M/s Star Bio Science. They were used without any treatment or purification. All other chemicals like trisodium citrate, ammonium persulphate were of laboratory grade.
Methods
Degumming of silk: To remove silk gum from the raw eri silk fabric, later on fabric was degummed at 90°C for 1 hour in an aqueous solution containing 20% soap and 2 g/L sodium carbonate at fabric to liquor ratio 1:20. Degummed fabric was washed using hot water and then cold washed and finally dried in air.
Application of maleic acid on silk: Pre-soaking of degummed silk fabric with ammonium persulphate solution of concentration 1% following an application of maleic acid monomers formulation on the pre-soaked silk fabric were performed separately by padding technique in a laboratory two bowl padding mangle. After two successive fabrics dipping in the maleic acid formulation, the pressure between the squeezing rollers was adjusted to enable an overall pick up of 100%. The pH of the monomer solution was adjusted at different specified levels with the use of the required dose of soda ash and caustic soda. The aqueous monomers formulation usually contained a known dose of trisodium citrate and chitosan. Finishing silk with maleic acid in the presence of chitosan also rendered the silk fabric intending to impart antimicrobial property into silk. The padded squeezed fabrics were subjected to drying in an oven at 95°C for 10-15 min. The dried fabrics were then cured at 140°C for 5 min. Untreated and maleic acid-treated silk fabrics were assessed for change of the properties as listed below following standard procedures.
Determinations of moisture regain and weight gain after treatment: Moisture regain of the initial and treated silk fabrics was determined following a standard procedure (Annual Book of ASTM Standards, 1974). For the determination of weight gain upon finishing treatments using maleic acid, the finished fabric samples were first soap washed and then extracted under reflux in a water bath for 8-10 hours successively using water to ensure removal of traces of unreacted maleic acid monomer along with polymeric maleic acid that remains unbound to the chain molecules of silk fabric samples. The extracted fabric samples were then oven-dried to a constant weight (W1) at 100°C. The weight gain (%) was then calculated based on the initial dry weight of degummed silk (W2), using the following relationship.
Tensile properties: The breaking strength of some selected fabric samples was measured in a Zwick 1445 CRT Universal Tensile Testing Machine, according to a method (Handbook of tensile testing, 1981) prescribed by IS: 1969-1968. The results obtained were based on an average of 10 tests in the warp direction of each sample. The test strip specimens were ravelled to a size of 50 mm × 20 mm between the jaw of the machine, and the test was performed with a traverse speed of 100 mm min-1 at a pretension of 0.5 N.
Determination of wrinkle recovery angle: Dry wrinkle recovery angle (warp+weft) of a selected fabric samples was determined using a SASMIRA Wrinkle Recovery Tester with specimen size of 25 mm × 20 mm following ASTM D1295-67 prescribed method.
Evaluation of antibacterial property of textile fabric: Antibacterial susceptibility testing was done using the well diffusion assay, as prescribed by the National Committee for Clinical Laboratory Standards. The antibacterial activity was quantitatively assessed against Gram-negative bacteria Klebsiella pneumoniae (K. pneumoniae ATCC 4352) and Gram-positive bacteria Staphylococcus aureus (S. aureus ATCC 6538), according to the AATCC 100-2004 test method. The fabric samples with 2.5 ± 0.1 cm in diameter were placed in a 250 ml glass jar with a screw cap and absorbed with 1.0 ± 0.1 ml of bacterial inoculum. Then 100 ml of sterilized saline water (prepared by dissolving 0.85 g of sodium chloride in 100 ml of distilled water) was added into the jar and then shaken for 24 hours in a shaker at 100 rpm. After incubation over contact periods of 24 hours the solution was then serially diluted. The diluted solution was placed on a nutrient agar and incubated for 24 hours at 37°C ± 2°C. Colonies of bacteria recovered on the agar plate were counted, and the percent reduction of bacteria (R) was calculated by the following equation:
Where, A is the number of bacterial colonies from the treated specimen after inoculation over 24 hours of contact period, and B is the number of bacterial colonies from the untreated specimen after inoculation at zero contact time.
IR spectroscopy: IR spectra of unmodified and selectively modified silk samples were obtained following KBr pellet technique by using the Perkin-Elmer FT-IR spectrometer. The dried fiber samples were crushed to a finer size up to 20 meshes before palletizing with KBr. Four KBr pellets contained about 1% powdered fibers as test specimens were prepared separately for unmodified eri silk and eri silk modified with maleic acid in the presence of different catalytic systems as specified (Das et al., 2011).
Results and Discussion
Role of dual catalyst
To study the role of esterification catalyst and free radical polymerization catalyst for pad-dry-cure technique of silk fabric with maleic acid, the silk fabric was treated with maleic acid in the absence of either of the two catalysts. The effect of dual catalyst on maleic acid cure of eri silk fabric is given in Table 1.
| Ammonium persulphate | Trisodium citrate | Application of pH | Weight gain % | Wrinkle recovery angle |
Tearing strength retention (%) | Breaking load (N cm-1) |
Elongation at break (%) |
Bending length (cm) |
|---|---|---|---|---|---|---|---|---|
| 0% | 10% | 7 | 6.63 | 218 | 74% | 32 | 14 | 1.4 |
| 1% | 0% | 7 | 8.58 | 207 | 80% | 41 | 19 | 1.4 |
| 1% | 10% | 7 | 9.67 | 258 | 91% | 42 | 13 | 1.4 |
| Silk untreated | - | - | 170 | 100% | 43 | 17 | 1.6 | |
In each experiment maleic acid dose levels were maintained at 10% (w/w). Treatment of silk fabric in presence of peroxodisulphate as the free radical polymerization catalyst only resulted in poor weight gain and wrinkle recovery angle with retention of a high order of tensile strength. Such effects appear to be the consequence of only graft copolymerization induced by ammonium peroxodisulphate (as shown in chemical reaction scheme 2.a. (i) and (ii), 2.b. and 2.c. in Figure 2 in the introduction section) and limited self-catalyzed esterification reaction effected only at a high temperature of drying and curing. Silk fabric finished with maleic acid in presence of only esterification catalyst also resulted in poor weight gain with only marginal improvement in wrinkle recovery angle with high retention of tensile strength inconsequent to the establishment of ester linkages under the influence of esterification catalyst (as shown in reaction scheme 1 in Figure 2) with limited thermally induced graft copolymerization of maleic acid in absence of free radical polymerization catalyst. Under the influence of two catalysts taken together (ammonium persulphate and trisodium citrate) for the maleic acid cure of silk, substantial weight gain and wrinkle recovery angle is achieved.
Retention of tensile strength, however, suffers for the maleic acid cure of silk under the influence of a dual catalyst system in our study. Results in Table 1 clearly show that retention or improvements in weight gain, wrinkle recovery angle, and tensile strength are optimal on pad-dry-iron-cure of silk with maleic acid under the influence of dual catalyst system.
Batching time and pH
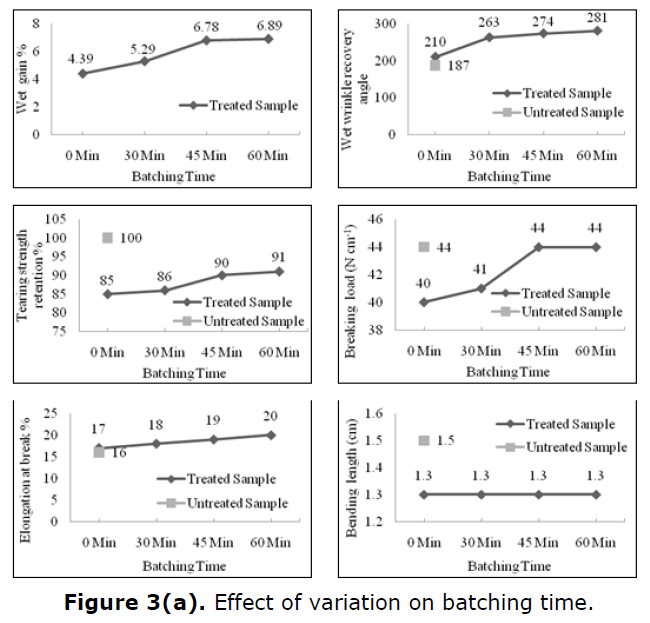
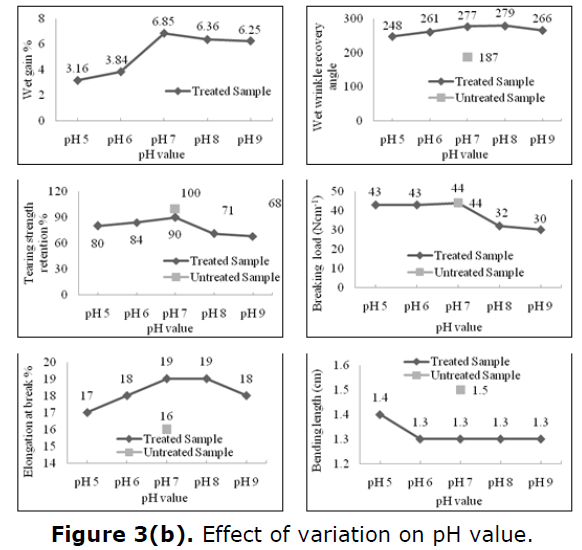
The effect of variation of batching time and pH on different physical and mechanical properties of degummed silk are shown in Table 2. In each experiment, the maleic acid dose level was maintained at 10% (w/w) for batching at room temperature 30°C for 60 min. In case of dual catalyst system and subsequent drying by heating at 95°C for 5 min, followed by curing at 140°C for 5 min, there is a notable weight gain, wrinkle recovery angle, tear strength retention, breaking load retention and elongation at break. However, bending length remained level for the entire period of batching time. The batching for an extended time distinctly favours a higher incorporation of maleic acid moieties on silk by ammonium persulphate induced graft copolymerization. Initial peroxy disulphate induced homo polymerization of maleic acid, to increase extents over increasing batching time periods, at ambient temperature 30°C and further polymerization of free maleic acid and silk-bound maleic acid moieties during the subsequent drying period at 95°C cause an overall change in environment and proximity of the hydroxyl groups of silk and carboxyl groups of the unbound or silk-bound maleic acid or poly (maleic) acid moieties in a manner that finally causes an enhanced degree of trisodium citrate catalyzed esterification and further chain polymerization leading to substantial crosslinking during curing at 140°C as revealed by the relevant data for wrinkle recovery in Table 2.
| Ammonium persulphate |
Trisodium citrate | Application of pH |
Weight gain % | Wrinkle recovery angle (w+f) |
Tearing strength retention (%) |
Breaking load (N cm-1) |
Elongation at break (%) | Bending length (cm) |
|
|---|---|---|---|---|---|---|---|---|---|
| Dry | Wet | ||||||||
| (A). Effect of variation of pH | |||||||||
| + | + | 5 | 3.16 | 218 | 248 | 80% | 43 | 17 | 1.4 |
| + | + | 6 | 3.84 | 228 | 261 | 84% | 43 | 18 | 1.3 |
| + | + | 7 | 6.85 | 252 | 277 | 90% | 44 | 19 | 1.3 |
| + | + | 8 | 6.36 | 256 | 279 | 71% | 32 | 19 | 1.3 |
| + | + | 9 | 6.25 | 240 | 266 | 68% | 30 | 18 | 1.3 |
| + | - | 7 | 4.67 | 223 | 230 | 75% | 32 | 19 | 1.3 |
| - | + | 7 | 3.58 | 218 | 224 | 80% | 41 | 16 | 1.3 |
| (B). Effect of variation of batching time | |||||||||
| ++ | 0 | 7 | 4.39 | 193 | 210 | 85% | 40 | 17 | 1.3 |
| ++ | 30 | 7 | 5.29 | 228 | 263 | 86% | 41 | 18 | 1.3 |
| ++ | 45 | 7 | 6.78 | 251 | 274 | 90% | 44 | 19 | 1.3 |
| ++ | 60 | 7 | 6.89 | 258 | 281 | 91% | 44 | 20 | 1.3 |
| (C). Degummed silk | - | - | - | 174 | 187 | 100% | 44 | 16 | 1.5 |
Note: *Maleic acid: 10 %; Ammonium persulphate: 1%, Trisodium citrate: 6%, Chitosan-1%, Drying at 950C, Curing at 1400C for 5 min.
In each experiment, the maleic acid dose level was maintained at 10% (w/w) for batching at room temperature 30°C for 60 min. In the case of the dual catalyst system and subsequent drying by heating at 95°C for 5 min, followed by curing at 140°C for 5 min, there is a notable weight gain, wrinkle recovery angle, tear strength retention, breaking load retention and elongation at break. However, bending length remained level for the entire period of batching time. The batching for an extended time distinctly favours a higher incorporation of maleic acid moieties on silk by ammonium persulphate induced graft copolymerization. Initial peroxydisulphate induced homo polymerization of maleic acid, to increase extents over increasing batching time periods, at ambient temperature 30°C and further polymerization of free maleic acid and silk-bound maleic acid moieties during the subsequent drying period at 95°C cause an overall change in environment and proximity of the hydroxyl groups of silk and carboxyl groups of the unbound or silk-bound maleic acid or poly (maleic) acid moieties in a manner that finally causes an enhanced degree of trisodium citrate catalyzed esterification and further chain polymerization leading to substantial crosslinking during curing at 140°C as revealed by the relevant data for wrinkle recovery in Table 2.
The esterification reaction that assumes more prominence at the high processing temperature (140°C) in the final stage appears to be somewhat dependent on the initial batching time. Increase in batching time favours improved transformation of the grafted maleic acid/poly (maleic) acid units to ester moieties at the high curing temperature 140°C under the influence of the esterification catalyst in the final stage of processing. Optimum batching time (45-60) min also allows improved diffusion of finishing agent maleic acid within the chain molecules of silk. Effect of variation on batching time is given in Figure 3(a).
Relevant data for the change of pH indicate that under neutral condition pH 7, optimum grafting and esterification leading to muchimproved wrinkle angle and substantial weight gain are achieved with no loss of breaking strength and with more than 90% retention of tear initial fabric. In case of moderate acidic condition, pH 5-6, moderate improvement in extensibility with more than 80% retention of the tear strength of the initial fabric was achieved. Again, moderate alkaline conditions, pH 8-9 result in poor retention of breaking strength (<70%), and tear strength (<75%), despite substantial weight gain much as a consequence of weakening of the silk fiber in the fabric by alkali attack. Under slightly acidic conditions, pH 5-6, improvement in wrinkle recovery angle is comparatively poor even though tear strength and breaking strength retention are good, pH 7, therefore, apparently provides the most optimum condition of the finishing process. Effect of variation on pH is given in Figure 3(b).
The antibacterial activity was quantitatively assessed against Gram-negative bacteria viz Klebsiella pneumoniae (K. pneumoniae: Strain No ATCC 4352) and Gram-positive bacteria viz Staphylococcus aureus (S. aureus: Strain No ATCC 6538), according to the AATCC 100- 2004 test method. The bacterial reduction percentage of the treated eri silk fabric against K. pneumoniae (Gram-negative) and S. aureus (Gram-positive) on treated fabric is observed to be 92 and 94 respectively. The results of IR analysis suggest that esterification of maleic acid with the hydroxyl group of silk effectively accomplished in the presence of trisodium citrate and free radical polymerization of with simultaneous grafting of poly (maleic acid) on silk effective accomplished in presence of ammonium persulphate on pad-dry-cure of maleic acid-treated silk fabric. In the presence of two catalysts during maleic acid treatment both the reaction became prominent resulting crosslinking of protein molecules promoting wrinkle recovery of silk.
It is also noteworthy that since more crosslinking takes place when the reaction between the fibers and maleic acid is more active with a higher power or longer time, the treated fibers become hardened and straightened, resulting in a greater loss of tensile strength of fabrics. Furthermore, a long time enhances the hydrolysis of fibers in acid catalysts thereby reducing the tensile strength of the finished eri silk fabric. This work is aimed at establishing optimum conditions for the application of maleic acid evaluating attainable changes or improvements in the fabric nature and properties including crease-resistance, stiffness, strength, and moisture regain anti-microbial, properties. The results of such studies are reported in the present article.
Comparison of maleic acid with DMDHEU
Table 3 shows plots of weight gain, retention of breaking strength, crease-resistance, and moisture regain for application of different quantities of maleic acid under dual catalyst system at pH 7. It is indicated that optimum weight gain and balance of textile related properties for the finished fabrics are obtained for application of 10% maleic acid. A comparison between maleic acid finished and DMDHEU finished silk fabric against unfinished silk fabric concerning their property parameters are shown in Table 3. Maleic acid produces much desired improvement in the fabric quality maleic acid specially imparts a lower order of stiffness a higher retention of strength and higher moisture regain for a comparable improvement in wrinkle recovery angle.
| Types of reagent |
Wrinkle recovery angle (w+f) |
Tearing strength retention (%) | Moisture regain % | Bending length (cm) |
Tensile strength retention (%) |
Elongation at break |
|
|---|---|---|---|---|---|---|---|
| Dry | Wet | ||||||
| None | 174 | 187 | 100 | 10.1 | 1.4 | 100 | 16% |
| 10% maleic acid | 258 | 281 | 95 | 13.89 | 1.3 | 100 | 20% |
| 10% DMDHEU | 260 | 282 | 60 | 8.12 | 1.5 | 57 | 9.4% |
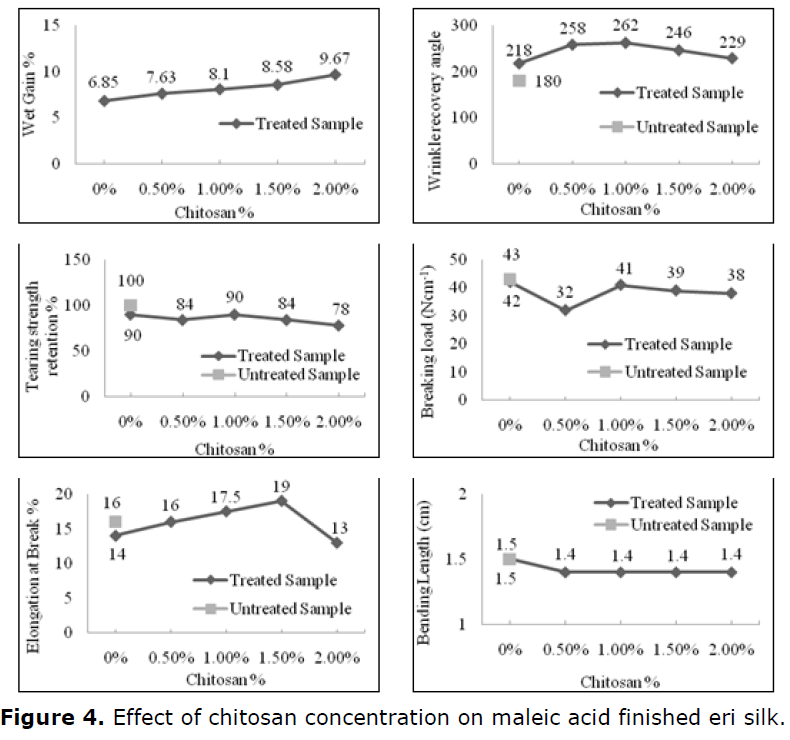
Effect of chitosan concentration
Figure 4 shows the effect of chitosan concentration (0.5-2 g/L) on the performance properties of eri silk, viz., wrinkle recovery angle, and tear and tensile strength of the treated fabric. The finishing baths were prepared containing ammonium persulphate 1% and trisodium citrate 6%, the fabrics treated thus with 100% pick up were dried, and then exposed to curing at 140°C for 5 min. It is clear from Figure 4 that the wrinkle recovery angle of the treated fabrics which was cured was pronounced as chitosan concentration increased up to 1 g/L and then decreased sharply whereas, there was a notable increase in weight gain with the increase of dose level of chitosan concentration. The enhancement in the wrinkle recovery angle of the finished fabrics by increasing chitosan concentration suggests that chitosan performed two functions: (1) it reacts with maleic acid in the fiber molecules; (2) chitosan undergoes crosslinking with the fabric to form a network matrix. The water-soluble chitosan with its low molecular weight penetrates the fiber more easily promoting anti creasing in the treated eri silk fabrics. Water-soluble chitosan generates ether reaction with the hydroxyl groups in the fibers, forming a two-dimensional structure that improved the crease resistance of the fabrics. Decrement in wrinkle recovery angle by increasing the chitosan concentration above 1 g/L could be associated with increased basicity of the finishing environment at higher chitosan concentrations. It is logical that basicity would stand as an inverse function to the acidity of the catalytic system of the crosslinking peptide molecule with maleic acid under the dual catalytic influence. Lower catalysis would certainly lead to decreased wrinkle recovery angle. With respect to tensile strength on the other hand, penetration or encapsulation of chitosan molecules would improve the strength properties of the treated fabrics. As shown in Figure 4, the tensile strength and elongation at break increased by increasing chitosan concentration up to 1 g/L which tends to decrease thereafter. Rigidity conferred on the structure of silk by the inclusion of chitosan through various interactions with silk and maleic acid may account for the decrease in tensile strength at higher chitosan concentrations and also, tear strength retention shows a monotonic fall with increases of chitosan dose level. It is also probable that higher concentrations of chitosan create more fibers bridging and are more likely to cause stress accumulation thereby decreasing the tensile strength. Breaking load increased by increasing chitosan concentration up to 1g/L which tends to decrease thereafter. Rigidity conferred on the structure of silk by the inclusion of chitosan through various interactions with silk and maleic acid may account for the decrease in tensile strength at higher chitosan concentrations and also, tear strength retention shows a monotonic fall with increases of chitosan dose level. It is also probable that higher concentrations of chitosan create more fibers bridging and are more likely to cause stress accumulation thereby decreasing the tensile strength.
Evaluation of antibacterial property of textile fabric
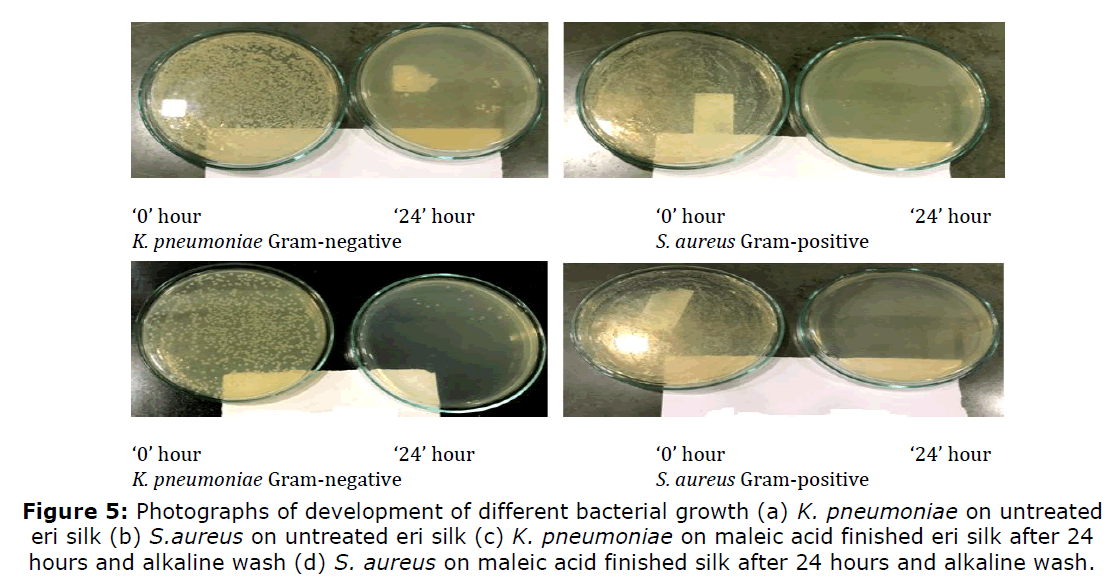
The antibacterial activity was quantitatively assessed against Gram-negative bacteria Klebsiella pneumoniae (K. pneumoniae: Strain No. ATCC 4352) and Gram-positive bacteria Staphylococcus aureus (S. aureus: Strain No. ATCC 6538), according to the AATCC 100-2004 test method. The photographs of bacterial growth on untreated and maleic acid treated samples in the presence of chitosan under dual catalytic effect are also given in Figure 5. Maleic acid treatment when suitably done with chitosan causes a substantial reduction in the growth of microorganisms in treated samples assessed in terms of colonies recovered. The finished is normally tested for antibacterial properties (AATCC 100 Test method) and finish stability is tested after 24 hours by washing the fabric with non-ionic detergent at mild alkaline pH. The results of the same are given in Table 4.
Figure 5. Photographs of development of different bacterial growth (a) K. pneumoniae on untreated eri silk (b) S.aureus on untreated eri silk (c) K. pneumoniae on maleic acid finished eri silk after 24 hours and alkaline wash (d) S. aureus on maleic acid finished silk after 24 hours and alkaline wash.
| Sample identification | Test culture | No. of colonies recovered at ‘0’ hr (B) |
No of colonies recovered at ‘24’ hrs after alkaline washing [A] | Reduction of microorganisms [R] |
|---|---|---|---|---|
| Silk and maleic acid | S. aureus | 1.96 × 10^5 | 1.8 × 10^4 | 90.81% |
| K. pneumoniae | 1.96 × 10^5 | 2.1 × 10^4 | 89.28 % | |
| Silk and maleic acid in | S. aureus | 2.00 ×10^5 | 1.2 × 10^4 | 94.00% |
| presence of catalysts | K. pneumoniae | 2.04 × 10^5 | 1.6 × 10^4 | 92.15% |
From the result, it can be inferred that the eri silk fabric finished with chitosan showed 99% antimicrobial property against both the bacteria.
IR analysis
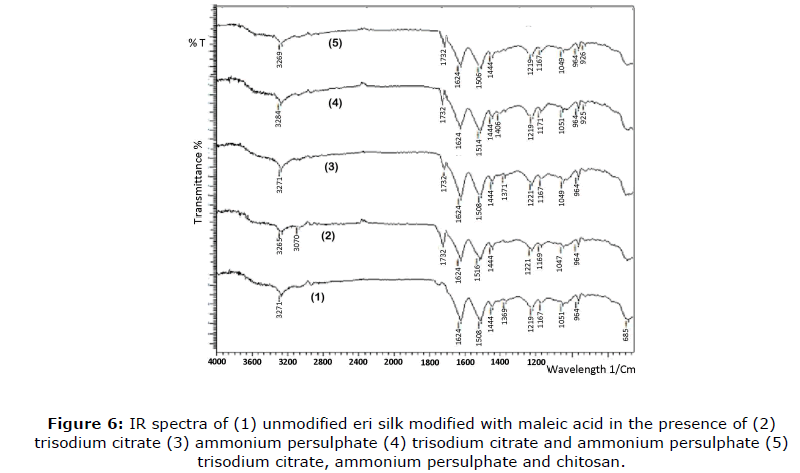
The Fourier Transform Infrared (FTIR) Spectra of untreated silk fabric and eri silk treated with maleic acid and chitosan under conventional curing are shown in Figure 6. A broad absorption band over 3400-3000 cm-1 characteristic of hydrogen-bonded (N-H) stretching vibration and an absorption band at 1624 cm-1 characteristic of amide stretching are common to all spectra (Das et al., 2014). Two notable absorption bands at 1508 cm-1 and 1444 cm-1 appearing in different intensities in the spectrum of unmodified silk (spectrum 1) are characteristic of carboxylate anion stretching and phenolic (-OH) bending, respectively. Carboxylate anion stretching accounts for the presence of a free carboxylic acid group at the end of polypeptide chains and phenolic (-OH) bending accounts for the presence of residues of tyrosine fractions of amino acids in the unmodified silk. A strong absorption band at 1219 cm-l also appears in the spectrum of unmodified silk and is attributed to (C-N) vibration of amine groups present at the end of polypeptide chains of silk. In the spectrum of unmodified silk, the absorption band at 1732 cm-1 (ester stretching) and 964 cm-1 (vinyl unsaturation) are practically not existent.
Maleic acid finishing of eri silk in the presence of ammonium persulphate catalyst only under the influence of only esterification catalyst trisodium citrate (spectrum 3 in Figure 6) results in the appearance of a strong absorption band 1508 cm-1 for carboxylate anion. The appearance of a new weak absorption band at 1732 cm-1 is occurred due to ester stretching vibration (spectrum 2 in Figure 6). The maleic acid finish on eri silk in the presence of esterification catalyst i.e. trisodium citrate only however results in intensification of the absorption band at 1221 cm-1 characteristic of vinyl ester stretching, substantial weakening of absorption band at 1508 cm-1 characteristic of unsaturation present in the vinyl group as expected. However, maleic acid finish on eri silk under the influence of dual catalyst system (spectrum 4 of Figure 6) results in weakening of the band at 964 cm-1 due to significant disappearance of the vinyl group unsaturation during final stage polymerization induced by heat and catalyst action along with the sharp intensification of the band at 1624 cm-1 due to stretching with retention of the band corresponding to 1508 cm-1 for carboxylate (anion) stretching. Silk treated with maleic acid and chitosan (spectrum 5) exhibited a decrease in absorbance intensity at 1624 cm-1 and 1508 cm-1 after the curing method as compared with the untreated silk sample. A decrease in intensity at 1624 cm-1 and 1508 cm-1 could be attributed to a decrease in the total number of hydroxyl groups through crosslink formation between silk and maleic acid. Substantial weakening/disappearance of band 1444 cm-1 corresponded to phenolic (-OH) bending due to the significant disappearance of phenolic (-OH) groups.
Silk proteins are known to attach to chitosan through carboxylate ions which shows antimicrobial potential. The results of the IR analysis are in tune with the mechanism proposed.
Conclusions
The appropriate maleic acid finish on silk in the presence of chitosan under neutral condition establishes a formaldehyde-free route for achieving simultaneous core and surface modification of silk with high scope for the incorporation of much improved physical and mechanical properties for the fabric.
The major advantages that can be derived from the maleic acid finish by following maleic acid pad-dry-cure technique under the dual catalytic influence of trisodium citrate and ammonium persulphate are substantial improvements in (i) wrinkle recovery, (ii) extensibility and (iii) moisture regain, (iv) anti-microbial properties with associated retention of a high order of tensile and tear strength.
References
- Alonso D, Gimeno M, Olayo R, Vázquez-Torres H, Sepúlveda-Sánchez JD, Shirai K (2009). Crosslinking chitosan into UV-irradiated cellulose fibres for the preparation of antimicrobial-finished textiles. Carb. Polym. 77(3):536-543.
- Arai T, Freddi G, Colonna GM, Scotti E, Boschi A, Murakami R, Tsukada M (2001). Absorption of metal cations by modified B. mori silk and preparation of fabrics with antimicrobial activity. J. Appl. Polym. Sci. 80(2):297-303.
- Bang SE, Lee ES, Kim SI, Yu YH, Bae SE (2007) Durable antimicrobial finish of silk fabrics. J. Appl. Polym. Sci. 106:938-943.
- Brodmann GL (1990). Performance of nonformaldehyde cellulose reactants. AATCC Review, 22(11):13-16.
- Cao W (2000). Demerits involved in nonformaldehyde finishing of common polycarboxylic acids and discussing of the corresponding solutions. Colour. Ann. 81:81-86.
- Das B, Padaki NV, Jagannathan K, Hubballi B, Naik SV (2017). Studies on handle behaviour of eri silk/wool blended fabrics developed for winter wear application. Procedia. Eng. 200:53-60.
- Das D, Datta DB, Bhattacharya P (2014). Simultaneous dyeing and finishing of silk fabric with natural color and itaconic acid. Cloth.Text. Res. J. 32(2):93-106.
- Das D, Datta DB, Bhattacharya P (2015). Concurrent dyeing and finishing of cotton fabric with natural colour and maleic anhydride. Int. J. Recent. Res. Phys. Chem. Sci. 1(2):13-23.
- Das D, Mukherjee A, Bhattacharya P, Chakrabarty D (2011). Finishing of silk by acrylic acid in the presence of sodium citrate and potassium persulphate as catalysts under thermal treatment. J. Appl. Polym. Sci. 121(2):770-776.
- Das D, Munshi R (2006). Finishing of cotton by methacrylic acid in presence of NaH2PO4 and K2S2O8 as catalysts under thermal treatment. J. Text. Inst. 97(6):519-525.
- Deshmukh G (2009). Ahimsa peace silk-an innovation in silk manufacturing. Man-Made Textiles in India, 52(12):421-424.
- El-Shafei AM, Fouda MMG, Knittel D, Schollmeyer E (2008). Antibacterial activity of cationically modified cotton fabric with carboxymethyl chitosan. J. Appl. Polym. Sci. 110(3):1289-1296.
- El-Tahlawy KF, El-Bendary MA, Elhendawy AG Hudson SM (2005). The antimicrobial activity of silk fabrics treated with different crosslinking agents and chitosan. Carb. Polym. 60:421-430.
- Kang I S, Yang C Q, Wei W, Lickfield GC (1998). Mechanical strength of durable press finished silk fabrics: Part I: Effects of acid degradation and crosslinking of cellulose by polycarboxylic acids. Text. Res. J. 68(11):865-870.
- Lee WK, Sin KM (1991). Silk fabric crosslinking. Text. Asia. 12:86-91.
- Lim SH, Hudson SM (2004). Application of a fibre-reactive chitosan derivative to cotton fabric as an antimicrobial textile finish. Carb. Polym. 56(2):227-234.
- Munshi R, Das D, Chowdhuri A (2014). Finishing of jute by polyacrylic rubber. J. Text. Inst. 105(1):67-73.
- Munshi R, Majumdar S (2018). Efficacy of microwave curing on water-soluble chitosan treated eri silk fabric to impart antibacterial and easy-care properties, Sericologia. 58(3and4):160-171.
- Shin Y, Min K (1996). Antimicrobial finishing of silk fabrics with chitosan (I)-Effect of degree of deacetylation on the antimicrobial property. J. Korean. Fibre. Soc. 33:487-491.
- Shirvan AR, Shakeri M, Bashari A (2019). Recent advances in the application of chitosan and its derivatives in the functional finishing of textiles. In The Impact and Prospects of Green Chemistry for Text. Tech. 107-133.
- Sudarshan NR, Hoover DG, Knorr D (1992). Antibacterial action of chitosan. Food. Biotech. 6(3):257-272.
- Tsai GJ, Su WH (1999). Antibacterial Activity of Shrimp Chitosan against Escherichia coli. J. Food. Prot. 62(3):239-243.
- Yang Y, Li S (1992). Crease-proofing silk. Text. Asia. 23(7):72-75.