Research Article - (2022) Volume 10, Issue 2
Imaging of mitral valve area by cardiac magnetic resonance in patients with rheumatic mitral stenosis
Ahmed Ibrahim Bedier1* and Amal Sakrana22Department of Diagnostic and Interventional Radiology, Mansoura University Hospital, Mansoura University, Egypt
Received: 20-May-2022, Manuscript No. MACR-22-61171; Editor assigned: 25-May-2022, Pre QC No. MACR-22-61171 (PQ); Reviewed: 14-Jun-2022, QC No. MACR-22-61171; Revised: 22-Jun-2022, Manuscript No. MACR-22-61171 (R); Published: 30-Jun-2022, DOI: 10.51268/2736-1888.22.10.137
Abstract
Background: Rheumatic heart disease which is a result of rheumatic fever is still a major health problem in developing countries. Rheumatic Mitral Stenosis (MS) is the commonest delayed valvular affection as a consequence of rheumatic fever. The assessment of MS severity by measuring Mitral Valve Area (MVA) is very essential for patient management. Different imaging modalities are available for MVA assessment including echocardiography and Cardiovascular Magnetic Resonance (CMR).
Objectives: The aim of this study is to compare the planimetric MVA between CMR and two dimensional echocardiography in MS patients.
Patients and methods: A forty adult patients with symptomatic mitral stenosis were included in the study. Other significant valvular lesions, atrial fibrillation, poor echocardiographic window, contraindications to CMR, and NYHA IV were excluded from the study. All patients were assessed by 2D echocardiography and CMR for MVA measurement.
Results: the mean 2D TTE MVA was 1.2 ± 0.26 cm2, while the mean CMR MVA 1.2 0.28 cm2. No significant statistical difference was found between both methods (P value 0.842) with a very strong correlation between both methods (r=0.93 and p-value <0.0001). The mean difference of MVA between the two methods was 0.012 cm2.
Conclusion: CMR is a non-invasive imaging modality that provides MVA measurement and is a reliable method in the diagnosis of MS patients.
Keywords
Rheumatic heart disease, Cardiovascular magnetic resonance, Mitral stenosis, Echocardiography, Mitral valve area.
Introduction
Rheumatic heart disease which is a result of rheumatic fever that is caused by group A beta hemolytic streptococci, is still a major health problem in developing countries. Although the health related burden of rheumatic heart disease has been declined worldwide, it is still considered a cause of young population morbidity and mortality (Woldu B et al., 2016; Watkins DA et al., 2017; Sohrabi B et al., 2018 ).
Rheumatic Mitral Stenosis (MS) which is defined as diastolic narrowing of the Mitral Valve (MV) orifice is the commonest delayed valvular affection as a consequence of rheumatic fever. Characteristic changes that occur in mitral valve as a result of rheumatic fever are leaflet edges thickening, commissural fusion, and chordal thickening, shortening and fusion (Lilly ES et al., 201 2).
Mitral stenosis diagnosis and assessment of its severity are very essential for timing and selection of the method of treatment. Echocardiography represents the corner stone for diagnostic assessment in patients with MS by assessment of Mitral Valve Area (MVA) and is also helpful in selection of patients for valvuloplasty (Lancellotti P et al., 2017).
Although echocardiography is the main imaging modality used to assess MS severity, Cardiovascular Magnetic Resonance (CMR) also may be performed as a complementary noninvasive technique especially, when TTE and TEE evaluations are of suboptimal quality and in whom Doppler studies are inconsistent with the clinical data. CMR has the advantage of being non-invasive, reproducible, not limited by air and bone conduction, more sensitive in detecting thrombus, and has a high 3D spatial resolution and freedom of access to any location in any position. (Helvacioglu F et al., 2014).
To date, no studies have been done on the role of CMR in evaluating rheumatic Mitral Stenosis (MS) in Egypt and comparing the results with echocardiographic results.
Objective of the study
The aim of this study is to compare the planimetric MVA between CMR and two dimensional echocardiography in patients with MS.
Patients and Methods
This prospective study was carried out between January 2019 and January 2020 on forty adult patients with severe symptomatic MS admitted to Specialized Medical Hospital, Cardiovascular Medicine Department, and Mansoura University and referred to Magnetic Resonance Unit, Radiology Department, and Mansoura University Hospital for CMR. Patients with other significant valvular lesions, atrial fibrillation, poor echocardiographic window, contraindications to CMR, and NYHA IV were excluded from the study.
Written consent was obtained from all patients and the study was accepted by IRB committee.
A detailed history including age, sex, residence, NYHA class, history of previous commisurotomy or PBMV, antibiotic prophylaxis, anticoagulants, and previous history of TIA, stroke or embolization. A detailed physical examination was done also.
The study included 40 patients, 5 males (12.5%) and 35 females (87.5%). The age ranged from 24 to 60 years with a mean of 37.3 ± 8.4 years. As regard presence of other medical disease; 38 patients (95%) had no medical diseases while one patient (2.5%) had diabetes mellitus and another one had chronic kidney disease (2.5%). All patients were from rural areas in Dakahlia and neighboring governorates (Table 1).
| Age/y | Mean ± SD (min-max) | |
|---|---|---|
| 37.3 ± 8.4 (24-60) | ||
| Sex | (N %) | |
| Male | 5 | 12.5 |
| Female | 35 | 87.5 |
| Medical disease | ||
| No | 38 | 95 |
| Yes DM | 1 | 2.5 |
| Yes CKD | 1 | 2.5 |
| Residence | ||
| Rural | 40 | 100 |
Echocardiography
It was done by (GE vivid E9 XDclare, GE Medical Systems, General Electric Company, Manufacturer GE Vingmed Ultrasound AS, Horten, Norway), with the probe (4V Hz). Assessment of mitral valve morphology was done in parasternal long axis, short axis and Apical views, with evalultion of subvalvular apparatus, MV leaflets mobility, thickness and calcification. Assessment of Mitral Valve Area (MVA) by MV plannimetry was performed in short-axis view, in the diastolic frame with maximum diastolic opening of the MV, with identifying the smallest orifice at the leaflet tips. Estimation of MVA less than 1.5 cm2 is considered an indication for intervention according to the latest ESC guidelines (Baumgartner H et al., 2018 ).
CMR assessment
CMR was done by a clinical MR scanner 1.5-T scanner, Philips, Ingenia. During CMR study the procedure was explained to the patient with training for breath hold technique with continuous monitoring of the patient heart rate. A 16-channel torso phased-array receiver coil was used for signal reception. All acquisition of data was retrospective ECG gated and with respiratory gating. The scan protocol was carried out in the following order:
• A Scout images (axial, coronal, sagittal) using Real-time interactive planning (FOV 450 × 450, mm2, slice thickness 10 mm acquisition matrix 220 × 176, voxel size 1.6 mm × 1.9 mm × 10 mm, echo/repetition time (TE/TR) shortest and flip angle 50°).
• Cine Steady State Free Precession (SSFP) sequences were acquired on the long axis cardiac planes: 4 chambers, 2 chambers, and 3 chambers (FOV 350 × 350 mm2, slice thickness 6-8 mm, acquisition matrix 220 × 176, voxel size 1.7 mm × 1.7 mm × 10 mm, and flip angle 60°), followed by a “stack” of contiguous SSFP cine images, with the same technical parameters, acquired along cardiac short axis, to cover the whole LV from base to apex.
• The MV was visualized with 4 chamber, 2 chamber and 3 chamber views. Short axis LV images were performed parallel to the mitral valve plane; 4–6 cross sections were obtained (with the same previous parameters but with slice thickness of 5 mm, with 5-6 slices, with slice gap-1). Then the minimal diastolic area was chosen as the planimetric MVA.
• Depending on the heart rate, and patient ability to hold breath the average scanning time was 10-20 minutes.
CMR imaging analysis
• Cardiac morphology and function were quantitatively evaluated on the cine images with the workstation (Circle CMR cvi42 cardiovascular imaging Inc 2016, Calgary, Canada). Two CMR radiologists assessed the CMR findings independently and parameters were recorded. The CMR images were assessed for mitral valve morphology, motion, and thickness. Calculation of planimetric MVA was done by measuring the smallest orifice at mid diastole in different slices.
• All CMR data were blinded to echocardiographic data.
Statistical analysis
The history, 2D data, and CMR data were recorded on an investigation report form, and tabulated, coded then analyzed. All statistical analyses were performed using SPSS (statistical package of social sciences) version 22 for windows (SPSS Inc., Chicago. IL, USA). Normality of data was first tested by Shapiro-Wilk test. Parametric data were presented as mean ± Standard Deviation (SD), while non-parametric data were expressed in median, minimum and maximum. Categorical data were presented as absolute numbers and percentage (%). Correlation analysis between echocardiography and CMR was done by Pearson coefficient of correlation test. Scatter plot graphs were used to represent the significant correlation. A 2tailed P-values ≤ 0.05 were considered to be statistically significant, and ≤ 0.001 were considered to be statistically highly significant. The smaller the p-value obtained, the more significant are the results.
Results
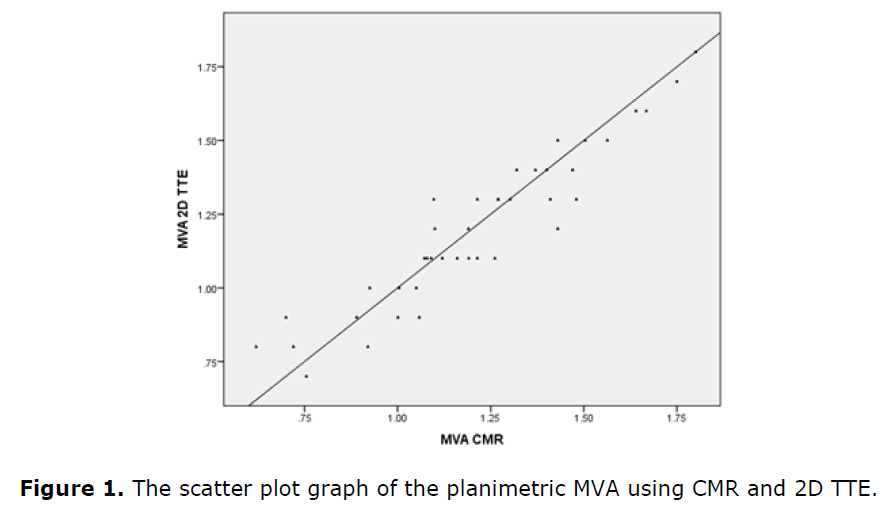
The study included forty MS patients; thirty three of them were NYHA II 33 (82.5%), while 6 patients (15%) were NYHA III, and only one patient was NYHA I (2.5%). Only one patient had history of closed commisurotomy (2.5%), and 7 cases had previous PBMV (17.5%). Only one patient had history of TIA (2.5%). 5 patients were on anticoagulation therapy (12.5%) while 35 patients were not on anticoagulation therapy (87.5%). 36 patients were on penicillin antibiotic prophylaxis (90%), while 4 patients did not receive penicillin antibiotic prophylaxis (10%) (Table 2 and 3 and Figure 1).
| Variable | Mean ± SD | |
|---|---|---|
| BSA | ||
| 1.94 ± 0.14 | ||
| NYHA | (N %) | |
| Class I | 1 | 2.5 |
| Class II | 33 | 82.5 |
| Class III | 6 | 15 |
| Previous commisurotomy | ||
| No | 39 | 97.5 |
| Yes,24y | 1 | 2.5 |
| Previous PBMV | ||
| No | 33 | 82.5 |
| Yes | 7 | 17.5 |
| Antibiotic prophylaxis | ||
| Yes | 36 | 90 |
| No | 4 | 10 |
| Oral anticoagulants | ||
| Yes | 5 | 12.5 |
| No | 35 | 87.5 |
| Previous stroke or TIA | ||
| No | 39 | 97.5 |
| Yes | 1 | 2.5 |
| 2D TTE | CMR | ||
|---|---|---|---|
| N | Valid | 40 | 40 |
| Missing | 0 | 0 | |
| Mean | 1.2 | 1.2 | |
| Median | 1.2 | 1.2 | |
| Std. Deviation | 0.26 | 0.28 | |
| Minimum | 0.7 | 0.62 | |
| Maximum | 1.8 | 1.8 | |
There was a significant positive correlation between both modalities in direct MVA planimetry measurement (P-value <0.0001).
The correlation had a high linearity between both modalities (r>0.9).
Direct planimetry of mitral valve area is the corner stone for diagnosis of mitral stenosis and for decision making as presence of a symptomatic patient with MVA less than 1.5 cm2 is an indication for intervention either with MV replacement or PBMV. Planimetric MVA can be assessed by different modalities. In our study we assessed MV by 2D TTE, and CMR. In our study; the mean 2D TTE MVA was 1.2 ± 0.26 cm2 (range 0.7-1.8 cm2), while the mean CMR MVA 1.2 ± 0.28 cm2 (range 0.62-1.8 cm2).
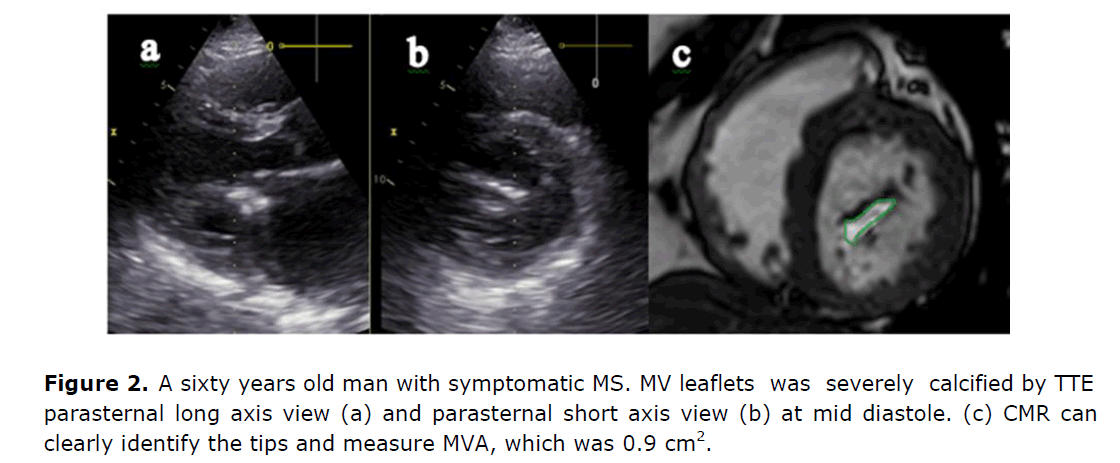
No statistically significant difference in MVA was found between the 2D TTE and CMR (P value 0.842) with a very strong correlation between both methods (r=0.93 and p-value <0.0001). The mean difference of MVA between both methods was 0.012 cm2, with slight overestimation of CMR MVA by 0.12%. In our study we observed the advantage of CMR in patients with heavily calcific valves; as MVA can be measured easily without limitation as in echocardiography (Figure 2).
This significant correlation between 2D TTE and CMR in this study is concordant with a study done by (Mutnuru P C et al. 2016); who performed 2DTTE and CMR on 50 Indian patients with different rheumatic valvular affection with MS was the predominant valve affection in the study. They found that the mean MVA by 2D TTE was 1.79 ± 0.43 cm2 and by CMR 1.82 ± 0.47 cm2 (r=0.98, p-value <0.00001) and they described a highly significant strong positive association between the results by 2D TTE and CMR.
Our results are also concordant with another study done by (Kim SS et al., 2015) on 102 MS patients with AF; where they compared MVA by TTE and PHT with MVA by CMR and MSCT. The mean MVA by 2D TTE was 1.16 ± 0.28 cm2 and CMR 1.15 ± 0.28 cm2; the correlation between CMR and TTE planimetry (r=0.67, P<0.05). The mean difference between the 2D TTE and CMR MVAs was 0.01 (P=0.61) with an overestimation of 0.9% by CMR.
Another CMR study done by (Helvacioglu F et al., 2014); who performed 2DTTE and CMR on 30 patients with rheumatic mitral stenosis in Turkey; the mean 2D TTE MVA in their study was 1.50 ± 0.53 cm2 and the mean CMR MVA was 1.50 ± 0.26 cm2. No statistically significant difference in MVA was found between the 2D TTE and CMR (P=0.90). A very strong correlation was found also between both methods in MVA assessment (r=0.971, P<0.0001). The mean difference of MVA between both methods was 0.018 cm2. Our results are also concordant with another study done by (Charan Lanjewar BE et al., 2010) on 30 patients with rheumatic mitral stenosis in India. The mean CMR MVA, was 1.71 ± 0.44 cm2 (range: 0.5-2.40 cm2); and the mean MVA by 2D TTE, 1.31 ± 0.30 cm2 (range: 0.6 to 2.50 cm2). The correlation between planimetric MVA by CMR and 2D TTE was very good (r=0.81, p<0.05). The mean absolute difference between both methods was 0.12 ± 0.23 cm2 (p<0.05), with a slight overestimation (by 7.6%) of the CMR MVA when compared to 2D TTE MVA by 8.1%.
Our study results and the previous studies mentioned before are also in line with a study done by (Djavidani B et al., 2005); where they compared MVA by CMR, 2D TTE, and invasively by Gorlin-formula at the catheterization laboratory in 22 patients with mitral stenosis in Germany. The correlation between planimetric CMR MVA and 2 D TTE MVA was very good (r=0.81, p<0.0001). The mean absolute difference between CMR MVA and 2 D TTE MVA was 0.13 ± 0.24 cm2 (p<0.05), resulting in a slight overestimation of CMR MVA as compared with 2D TTE MVA by 8.1%.
Limitation of the Study
• The small number of patients included in this study is considered a limitation, but other studies were done on also a smaller number of patients.
• Exclusion of AF in the study patients, however it is more common in MS patients, but the aim of exclusion of AF patients was to avoid averaging of measurements which may lead to bias.
• We didn’t compare our MVA results with Gorlin Formula (the gold standard method for MVA), because this invasive method is not done routinely for MS patients and restricted only in special circumstances where there is discrepancies between echocardiographic measurements and clinical status. However the Gorlin method is invasive and has several pitfalls and technical limitations (Hammermeister KE et al., 1973; Klarich KW et al., 1996).
• We used the conventional 2D planimetry method is the reference method because other Doppler methods including PHT, PISA, and continuity equation have their own limitations (Flachskampf FA et al., 1990; FredmanC S et al., 1990).
Discussion
Cardiovascular magnetic resonance is a noninvasive imaging modality that can provide a reliable assessment of MVA with comparable results with echocardiography.
Subacute thyroiditis is one of the common causes of thyroid pain, most often occurs at 40 to 50 years of age (Ross DS et al.,2016). In this case, clinical suspicion of subacute thyroiditis was made base on prodrome of fever and malaise followed by thyroid pain. Subacute thyroiditis generally presents after a viral upper respiratory tract infection, commonly within the past 30 days (Ross DS et al.,2016; Fatourechi e t al., 2003). CMV, Coxsackie virus, influenza virus, adenovirus, EBV, and herpes simplex virus have been reported in association with subacute thyroiditis (Desailloud R et al .,2009). Symptoms of pharyngitis occurred at the same time with thyroid pain in this case. Patient also experience hyperthyroid symptoms such as sweating, heat intolerance, palpitation, and on and off loose stool. Thyroid function test showed hyperthyroid status with elevated fT4 and suppressed TSH. Approximately 50% of patients with subacute thyroiditis have an initial phase of hyperthyroidism (Ross DS et al., 2016; Bindra A et al., 2008). This happens as the result of damaged thyroid follicular cells by activated cytotoxic T lymphocytes, causing unregulated release of preformed thyroid hormones into the circulation. The diagnosis of subacute thyroiditis was supported by raised ESR and CRP, as well as other imaging studies discussed below. Negative history of radiation and trauma to the neck area, with no recent intake of new drugs especially Amiodarone, interferon-alfa, interleukin-2, and lithium making the differential diagnosis of radiation, trauma or drug induced thyroiditis less likely (Bindra A et al., 2008).
Ultrasound of neck was performed to exclude presence of intrathyroidal collection or illdefined hypoechoic areas of low vascularization which is commonly seen in cases of acute suppurative thyroiditis (Takahashi MS et al ., 2019) Presentation of acute suppurative thyroiditis can mimic subacute thyroiditis with the presence of fever and swollen, tender thyroid, thus need to be excluded particularly in this case where patient is immune compromised. This is important as fine-needle aspiration of the lesion with parenteral antibiotics coverage is necessary in the case of acute suppurative thyroiditis, and surgical drainage may be required as well. In this case, intra thyroidal collection was not detected during ultrasound of the neck. There was no increase vascularity on color Doppler of thyroid gland, suggestive of subacute phase of thyroiditis, as acute phase of thyroiditis often shows diffuse hyper vascularization of thyroid gland (Bindra A et al., 2008). Thyroid scintigram on the other hand showed significant reduce tracer uptake within the thyroid region. Low radioiodine uptake in the context of hyperthyroid status is in favour of the diagnosis of subacute thyroiditis. In this case, postviral subacute thyroiditis is likely with the presence of pharyngitis (Ross DS et al .,2016; Mayer KH et al., 2007; Bindra A et al., 2008).
Beta blockers and anti-inflammatory therapy remains the mainstay of treatment for subacute thyroiditis ( Ross DS et al., 2016). T Propranolol 20mg BD was started to control thyrotoxic symptoms. T Ibuprofen was prescribed to alleviate pain in this case, which is also the first line therapy for subacute thyroiditis. Corticosteroid therapy should be initiated if patient fail to respond to full doses of No steroidal Anti-Inflammatory Drugs (NSAIDs) over several days. Fortunately, patient’s symptoms controlled with T Ibuprofen and he was discharged home uneventful on day 6 of admission. With NSAIDs, the median time for the resolution of pain is about 5 weeks. Unlike other etiologies of hyperthyroidism, antithyroid drugs have no role in the treatment of subacute thyroiditis (Ross DS et al., 2016). Hyperthyroidism state in subacute thyroiditis is usually transient, enduring for three to six weeks and ceasing when the thyroid stores are exhausted. During the follow up sessions in endocrine clinic, patient reported resolution of fever as well as significant reduction in thyroid pain and swelling. Clinically, he is euthyroid even though latest repeated thyroid function test shows reduced fT4, consistent with hypothyroidism which might happen in 34 to 60% of subacute thyroiditis patients within the first 12 months (Fatourechi V et al., 2003; Benbassat CA et al., 2007) Both ESR and CRP were normalized by week 7. Anyway, thyroid function tests need to be monitored continuously in successive clinic reviews, as permanent hypothyroidism develops in approximately 10% of patients with subacute thyroiditis, where by thyroxine replacement is required. Study by Takahashi MS et al. shows subacute thyroiditis patients with hypothyroid phase tend to have antithyroid antibodies at baseline (Benbassat CA et al., 2007). Both TRAb and TPO antibodies were absent in this case (Fatourechi V et al., 2003). Reported 4% of recurrence rate amongst patient with subacute thyroiditis.
Conclusion
This case highlights the importance of diagnosing subacute thyroiditis in patient presenting with hyperthyroidism, and tender, swollen thyroid gland from careful history taking, clinical examination, as well as imaging studies. Both ultrasound of neck and thyroid scintigram play a significant role in excluding other causes of thyroiditis. Beta blockers and anti-inflammatory therapy remains the mainstay of treatment for subacute thyroiditis, where antithyroid drugs has limited role in managing the transient thyrotoxicosis. Thyroid function tests should be performed from time to time in subsequent clinic visits to monitor complication of permanent hypothyroidism, where thyroxine replacement might be required.
Recommendations
CMR is a non-invasive imaging modality that can be used as an alternative to TTE in cases with poor acoustic windows or in calcific mitral valves.
References
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Munoz DR, Rosenhek R. ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2018;76(1):1-62.[Crossref] [Google Scholar] [PubMed]
Charan Lanjewar BE, Mishra N, Jhankariya B, Kerkar P. Planimetry of mitral valve stenosis in rheumatic heart disease by magnetic resonance imaging. J Heart Valve Dis. 2010 May;19(3):357-363. [Crossref] [Google Scholar] [PubMed]
Djavidani B, Debl K, Lenhart M, Seitz J, Paetzel C, Schmid FX, Nitz WR, Feuerbach S, Riegger G, Luchner A. Planimetry of mitral valve stenosis by magnetic resonance imaging. J Am Coll Cardiol. 2005 Jun 21;45(12):2048-2053. [Crossref] [Google Scholar] [PubMed]
Flachskampf FA, Weyman AE, Gillam L, Chun-Ming L, Abascal VM, Thomas JD. Aortic regurgitation shortens Doppler pressure half-time in mitral stenosis: clinical evidence, in vitro simulation and theoretic analysis. J Am Coll Cardiol. 1990 Aug 1;16(2):396-404. [Crossref] [Google Scholar] [PubMed]
Fredman CS, Pearson AC, Labovitz AJ, Kern MJ. Comparison of hemodynamic pressure half-time method and Gorlin formula with Doppler and echocardiographic determinations of mitral valve area in patients with combined mitral stenosis and regurgitation. Am Heart J. 1990 Jan 1;119(1):121-129. [Crossref] [Google Scholar] [PubMed]
Hammermeister KE, Murray JA, Blackmon JR. Revision of Gorlin constant for calculation of mitral valve area from left heart pressures. Br Heart J. 1973 Apr; 35(4):392. [Crossref] [Google Scholar][PubMed]
Helvacioglu F, Yildirimturk O, Duran C, Yurdakul S, Tayyareci Y, Ulusoy OL, Aytekin S. The evaluation of mitral valve stenosis: comparison of transthoracic echocardiography and cardiac magnetic resonance. Eur Heart J Cardiovasc Imaging. 2014 Feb 1; 15(2):164-169. [Crossref] [Google Scholar] [PubMed]
Kim SS, Ko SM, Song MG, Chee HK, Kim JS, Hwang HK, Lee JH. Quantification of stenotic mitral valve area and diagnostic accuracy of mitral stenosis by dual-source computed tomography in patients with atrial fibrillation: comparison with cardiovascular magnetic resonance and transthoracic echocardiography. Int J Cardiovasc Imaging. 2015 Jun;31(1):103-114. [Crossref] [Google Scholar] [PubMed]
Klarich KW, Rihal CS, Nishimura RA. Variability between methods of calculating mitral valve area: simultaneous Doppler echocardiographic and cardiac catheterization studies conducted before and after percutaneous mitral valvuloplasty. J Am Soc Echocardiogr. 1996 Sep 1;9(5):684-690. [Crossref] [Google Scholar] [PubMed]
Lancellotti P, Dulgheru R, Vannan M, Yoshida K. Heart valve disease (mitral valve disease): mitral regurgitation. The EACVI Textbook of Echocardiography. 2017 Jan 7;282. [Crossref] [Google Scholar]
Mutnuru PC, Singh SN, D’Souza J, Perubhotla LM. Cardiac MR imaging in the evaluation of rheumatic valvular heart diseases. J Clin Diagn Res. 2016 Mar;10(3):06. [Crossref] [Google Scholar] [PubMed]
Sohrabi B, Ranjbar A. Global burden of rheumatic heart disease. N Engl J Med. 2018 Jan 4;378:e2. Woldu B, Bloomfield GS.
Rheumatic heart disease in the twenty-first century. Curr Cardiol Rep. 18(10):1-1.[Crossref] [Googe Scholar] [PubMed]
Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT, Mayosi BM, Mensah GA, Nascimento BR. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. 2017 Aug 24;377(8):713-722. [Crossref] [Google Scholar][PubMed]
Woldu B, Bloomfield GS. Rheumatic heart disease in the twenty-first century. Curr Cardiol Rep. 2016 Oct;18(10):1-1. [Crossref] [Google Scholar] [PubMed]